Head
Agata Adamczyk, Prof., Ph.D. phone: +48 22 60 86 72, This email address is being protected from spambots. You need JavaScript enabled to view it.
Secretariat
Dorota Rycko, phone: +48 22 60 86 613, This email address is being protected from spambots. You need JavaScript enabled to view it.
Research staff
Joanna B. Strosznajder, Prof., Ph.D., MD., phone: +48 22 60 86 414, This email address is being protected from spambots. You need JavaScript enabled to view it.
Grzegorz A. Czapski, Assoc. Prof., Ph.D., phone: +48 22 60 86 600, This email address is being protected from spambots. You need JavaScript enabled to view it.
Magdalena Gąssowska-Dobrowolska, Assoc. Prof., Ph.D., phone: +48 22 60 86 420, This email address is being protected from spambots. You need JavaScript enabled to view it.
Anna Wilkaniec, Ph.D., phone: +48 22 60 86 600, This email address is being protected from spambots. You need JavaScript enabled to view it.
Magdalena Cieślik, Ph.D., phone: +48 22 60 86 420, This email address is being protected from spambots. You need JavaScript enabled to view it.
Ewelina Pałasz, Ph.D., phone: +48 22 60 86 420, This email address is being protected from spambots. You need JavaScript enabled to view it.
Gabriela Olech-Kochańczyk, Ph.D., phone: +48 22 60 86 420, This email address is being protected from spambots. You need JavaScript enabled to view it.
Ewelina Bielska, M.Sc. Ing., phone: +48 22 60 86 413, This email address is being protected from spambots. You need JavaScript enabled to view it.
Technical staff
Elżbieta Gawinek, M.Sc. phone: +48 22 60 86 413, This email address is being protected from spambots. You need JavaScript enabled to view it.
Post-doc
Piotr Wójcik, Ph.D., phone: +48 22 60 86 413, This email address is being protected from spambots. You need JavaScript enabled to view it.
PhD students
Marta Matuszewska, M.Sc. Ing., phone +48 22 60 86 413, This email address is being protected from spambots. You need JavaScript enabled to view it.
Agnieszka Banaszek, M.Sc. Ing., phone +48 22 60 86 413, This email address is being protected from spambots. You need JavaScript enabled to view it.
Research profile
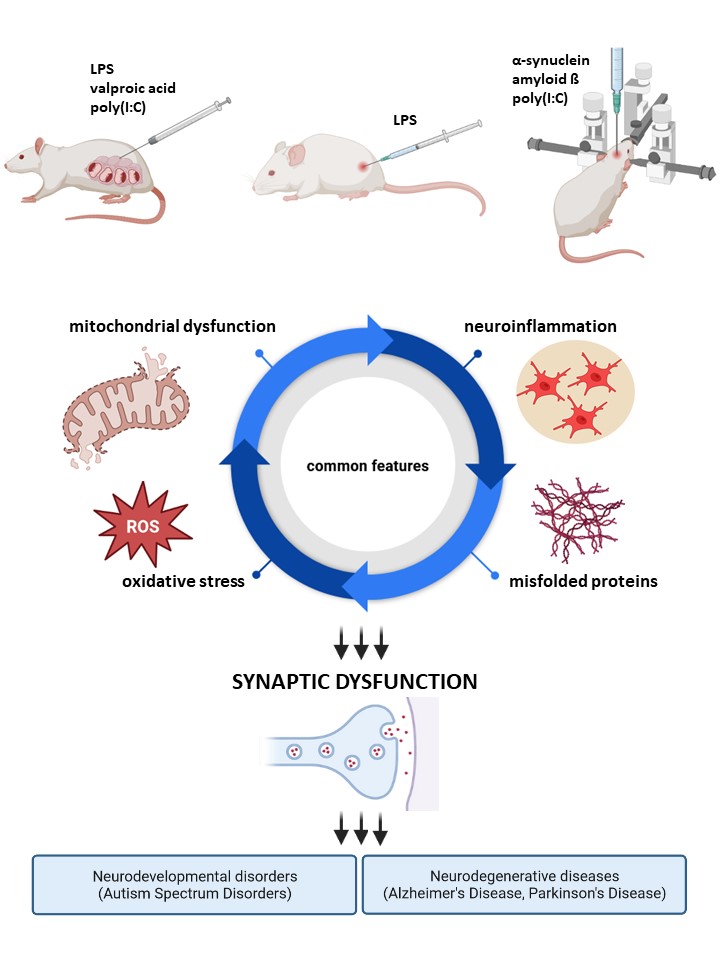
Our research interests are concentrated on the molecular mechanisms underlying neurodevelopmental disorders such as autism as well as neurodegenerative diseases, including Parkinson's and Alzheimer's disease. The pathomechanism of those syndromes is complex and the key molecular pathways involved are the immune system overactivation as well as the misfolding, aggregation, and accumulation of proteins (especially α-synuclein (α-syn) and amyloid beta (Aβ)) in the brain. We are focused on elucidating the relationship between those initiators and the following activation of glial cells, oxidative stress, and mitochondrial dysfunction in triggering synaptic pathology in the developing and mature brain. Our research is conducted in cooperation with leading domestic and foreign scientific centres, using modern research techniques and numerous experimental model systems of increasing complexity from the level of isolated organelles and cells, in (co-)cultures of neurons and glia, in organotypic brain slice cultures, in vivo animal models to the patient's tissue. Our overriding goal is to translate basic research into clinical applications to develop novel therapeutic strategies for the treatment of central nervous system disorders, leading to significant improvement in the lives of patients suffering from these devastating illnesses.
Grants
The mechanisms of mitochondrial damage-dependent neuroinflammation in experimental models of Parkinson’s disease. The role of Parkin dysfunction. NCN, OPUS 20 LAP, 2020/39/I/NZ4/01031, 2021-2025; Project manager – Prof. Agata Adamczyk, Ph.D.;
The impact of exercise on the level of the CDNF / MANF family neurotrophic factors in a murine model of Parkinson's disease induced by intracerebral administration of alpha-synuclein oligomers. NCN, MINIATURA 4, 2020/04/X/NZ4/00773, 2022-2023; Project manager - Ewelina Pałasz, PhD;
The role of ATP-dependent activation of P2 purinergic receptors in the energetic disturbances in the brain of autistic-like animals. NCN, Preludium 20, 2021/41/N/NZ4/02350, 2022-2025; Project manager - Lidia Babiec, M.Sc. Ing;
Novel role of peroxisome proliferator-activated receptor α in the regulation of amyloid β peptide metabolism and mitochondrial function in an animal model of Alzheimer’s disease. NCN Preludium 2019/35/N/NZ4/03706, 2020-2023; Project manager - Sylwia Wójtowicz, Ph.D.;
The role of BET family proteins in microglia-dependent neurodegeneration; relevance to Alzheimer's disease. NCN OPUS 2018/31/B/NZ4/01379, 2019-2023; Project manager – Grzegorz A. Czapski, Assoc. Prof., Ph.D.;
The influence of impairment of extracellular nucleoside and nucleotide signaling on synaptic structure and function in autism. NCN OPUS 2017/25/B/NZ4/01969, 2018-2022; Project manager – Prof. Agata Adamczyk, Ph.D.;
The role of maternal immune activation on mitochondria function. Implication for neurodevelopmental disorders. NCN SONATA 2016/23/D/NZ4/03572, 2017-2021; Project manager - Magdalena Cieślik, Ph.D.;
Molecular mechanisms involved of the gut-brain interaction in autism spectrum disorders. Potential role of probiotics in the treatment of autism. SymbioPharm GmbH, Germany; 2018-2019; Project manager – Agata Adamczyk, Assoc. Prof., Ph.D.;
Significance of sphingosine kinase-1 and sphingosine-1-phosphate in the experimental model of Parkinson's disease and pharmacological neuroprotection. NCN, Etiuda 4, 2016/20/T/NZ3/00504, 2016-2017; Project manager – Joanna A. Motyl, Ph.D.;
The role of Sphingosine -1-phosphate (S1P) receptors signaling in animal model of Parkinson’s disease. Searching of novel therapeutic targets. NCN, 2013/09/N/NZ4/02045, 2014-2016; Project manager - Joanna A. Pyszko, M.Sc.;
The role of poly(ADP-ribose) polymerase and sirtuins in molecular mechanism of cell death. Identification of novel targets for Alzheimer's therapy. NCN, OPUS, 2013/09//B/NZ3/01350, 2014-2017; Project manager – Prof. Joanna B. Strosznajder, Ph.D.;
The involvement of P2 purinergic receptors in alpha-synuclein mediated mitochondrial dysfunction. Relevance to Parkinson’s disease. NCN, 2013/009/D/NZ3/01359, 2014-2016; Project manager - Anna Wilkaniec, Ph.D.;
The role of Parkin in exogenous Alpha-synuclein-induced cell death. Implications for pathogenesis of Parkinson’s disease and other synucleinopathies. NCN, 2012/05/B/NZ3/02047, 2012-2016; Project manager – Agata Adamczyk, Assoc. Prof., Ph.D.;
Collaboration
- Medical University of Warsaw, Faculty of Pharmacy, Department of Pharmacodynamics, Warsaw, Poland - Prof. Magdalena Bujalska-Zadrożny;
- Nencki Institute of Experimental Biology PAS, Laboratory of Behavioral Methods, Warsaw, Poland - Paweł Boguszewski, Ph.D.;
- Nencki Institute of Experimental Biology PAS, Department of Biochemistry, Warsaw, Poland - Krzysztof Nieznański, Ph.D.;
- Pomeranian Medical University, Department of Biochemistry and Medical Chemistry, Szczecin, Poland - Prof. Irena Baranowska-Bosiacka;
- Central Clinical Hospital of the Ministry of Interior and Administration in Warsaw, Poland - Kamila Bojakowska, M.D.;
- Faculty of Chemistry, University of Warsaw, Poland - Marcin Strawski, Ph.D.;
- Medical University of Białystok, Poland - Prof. Barbara Mroczko;
- Institute of Pharmacology and Clinical Pharmacy, Biochemical and Pharmacological Center, Marburg, Germany - Prof. Carsten Culmsee;
- Louisiana State University Health Sciences Center, Department of Neurology, New Orleans, LA, USA - Prof. Walter J. Lukiw;
- University of Missouri, Thompson Center For Autism & Neurodevelopmental Disorders, Columbia , MO, USA - Prof. David Q. Beversdorf;
- University of Missouri, Department of Biochemistry, Columbia, MO, USA – Prof. Grace Y. Sun;
- Institute for Pharmacy and Biochemistry, Pharmacology and Toxicology, Johannes-Gutenberg University Mainz, Mainz, Germany - Prof. Kristina Friedland;
Research equipment
- Flow cytometer Becton Dickinson FACS Canto II;
- Microplate reader Thermo Scientific Multiscan GO;
- Spectrophotometer Nanodrop 2000;
- Spectrophotometer Shimadzu UV-1700;
- Thermocycler Perkin–Elmer GeneAmp PCR System 2400;
- Thermocycler Eppendorf Mastercycler;
- Inverted fluorescent microscope OLYMPUS IX-71;
- Scintillation counter Wallac 1409;
- Ultracentrifuge Backman LE-70;
- Stereotaxic instrument for small animals RWD Life Science;
- SomnoSuite Low-Flow Anesthesia System Kent Scientific;
Research methods
- analysis of muse and rat’s behavior using: open field test, novel object recognition test, social novelty test (Crawley’s test), social isolation test, elevated plus maze, rotarod performance test, grip strength test, pole test;
- cell culture (mammalian cells and bacteria);
- morphometric analysis of neurites in vitro;
- analysis of cell viability by MTT assay, LDH assay, trypan blue exclusion test, and after staining with propidium iodide;
- analysis of apoptosis-related chromatin condensation in vitro by Hoechst 33342 assay;
- analysis of the level of oxidative stress in vitro by using fluorogenic probes DCF, DAF-2 and MitoSOX;
- analysis of mitochondrial membrane potential in vitro by using JC-1 assay;
- analysis of ATP level in vitro by bioluminescence assay;
- analysis of the level of glutathione and the activity of redox state-related enzymes by using spectrophotometric method;
- analysis of lipid peroxidation and protein oxidation by spectrophotometric methods (TBARS, DNPH);
- analysis of calcium influx into cells in vitro by using radioisotope method or with fluorogenic probe FLUO-4;
- analysis of enzymes activity by radioisotope methods, spectrophotometric methods and fluorescence-based methods;
- analysis of protein immunoreactivity by Western blot, ELISA, LUMINEX, antibody array;
- analysis of post-translational protein modifications by immunoprecipitation followed by Western blotting (ubiquitynation, phosphorylation, S-nitrosylation, poly(ADP-ribosyl)ation);
- analysis of cytoskeleton stability by electrophoresis and immunoblotting of α/β-tubulin;
- immunohistochemistry;
- flow cytometry;
- isolation of nucleic acids (DNA, RNA, miRNA);
- analysis of the level of mRNA and miRNA by RT-PCR, qPCR, gene expression arrays;
- analysis of gene polymorphism by PCR and RFLP method;
- gene silencing in vitro with siRNA;
- cell transfection in vitro by electroporation or lipofection;
- transformation of bacteria using Ca2+ method;
- fluorescent and confocal microscopy;
- analysis of cell migration in vitro using scratch assay;
- analysis of phagocytosis in vitro by using fluorescent microspheres;
Experimental models in vitro:
- primary rat cortical neurons;
- cell line SH-SY5Y – neuron-like cells derived from human neuroblastoma;
- cell line C20 - immortalized human microglia;
- cell line LUHMES - Lund human mesencephalic cells;
- cell line PC12 – neuron-like cells derived from rat adrenal gland pheochromocytoma;
- cell line HT22 – immortalized mouse hippocampal neurons;
- cell line BV2 – immortalized mouse microglia;
- transfected cell line PC12 stably expressing human wild-type APP gene;
- transfected cell line PC12 stably expressing human APP gene with double Swedish mutation (K670M/N671L);
- transfected cell line PC12 stably expressing human wild-type SNCA gene;
- transfected cell line PC12 stably expressing human wild-type PRKN gene;
Experimental models in vivo:
- murine model of Alzheimer’s disease – intracerebroventricular administration of oligomeric Aβ;
- murine model of Parkinson’s disease – intrastriatal administration of α-synuclein;
- animal models of systemic inflammation – intraperitoneal administration of lipopolysacharide (LPS) to rats and mice;
- rat model of autism in offspring – prenatal exposition to valproic acid (VPA) – intraperitoneal administration of VPA at 12.5 day of pregnancy;
- rat model of neurodevelopmental diseases in offspring – prenatal exposition to maternal immune activation (MIA) – intraperitoneal administration of LPS at 9.5 day of pregnancy;
- murine model of neurodevelopmental diseases in offspring – prenatal exposition to maternal immune activation (MIA) – intraperitoneal administration of poly(I:C) at 17 day of pregnancy;
- Parkin knock-out mice (homozygous) – strain B6.129S4-Prkntm1Shn/J
- Parkin overexpressing mice - strain B6;FVB-Tg(Prnp-PARK2)196Kfw/EkraJ;
Selected publications
Babiec L., Wilkaniec A., Gawinek E., Hilgier W., Adamczyk A. Inhibition of purinergic P2 receptors prevents synaptic and behavioral alterations in a rodent model of autism spectrum disorders. Research in Autism Spectrum Disorders 2024, 112, 102353; https://doi.org/10.1016/j.rasd.2024.102353
Czapski GA, Matuszewska M, Cieślik M, Strosznajder JB. Inhibitor of bromodomain and extraterminal domain proteins decreases transcription of Cd33 in the brain of mice subjected to systemic inflammation; a promising strategy for neuroprotection. Folia Neuropathol. 2024;62(2):127-135. doi: 10.5114/fn.2024.138140
Ruiz-Ortega ED, Wilkaniec A, Adamczyk A. Liquid-liquid phase separation and conformational strains of α-Synuclein: implications for Parkinson's disease pathogenesis. Front Mol Neurosci. 2024 Oct 23;17:1494218. doi: 10.3389/fnmol.2024.1494218. PMID: 39507104; PMCID: PMC11537881
Gąssowska-Dobrowolska M, Olech-Kochańczyk G, Culmsee C, Adamczyk A. Novel Insights into Parkin-Mediated Mitochondrial Dysfunction and "Mito-Inflammation" in α-Synuclein Toxicity. The Role of the cGAS-STING Signalling Pathway. J Inflamm Res. 2024 Jul 11;17:4549-4574. doi: 10.2147/JIR.S468609. PMID: 39011416; PMCID: PMC11249072.
Żulińska S, Strosznajder AK, Strosznajder JB. Current View on PPAR-α and Its Relation to Neurosteroids in Alzheimer's Disease and Other Neuropsychiatric Disorders: Promising Targets in a Therapeutic Strategy. Int J Mol Sci. 2024 Jun 28;25(13):7106. doi: 10.3390/ijms25137106. PMID: 39000217; PMCID: PMC11241121
Żulińska S, Wencel PL, Czubowicz K, Strosznajder JB. Alterations in mRNA level of proteins related to redox state and mitochondria in Alzheimer’s disease animal model: Promising targets in neuroprotection Folia Neuropathol. 2024;62(3):237-247. doi: 10.5114/fn.2024.143039. PMID: 39529534
Babiec L, Wilkaniec A, Gawinek E, Hilgier W, Adamczyk A. Inhibition of purinergic P2 receptors prevents synaptic and behavioral alterations in a rodent model of autism spectrum disorders. Elsevier. Research in Autism Spectrum Disorders 112, 2024, 102353
Gąssowska-Dobrowolska M, Czapski GA, Cieślik M, Zajdel K, Frontczak-Baniewicz M, Babiec L, Adamczyk A. Microtubule Cytoskeletal Network Alterations in a Transgenic Model of Tuberous Sclerosis Complex: Relevance to Autism Spectrum Disorders. Int. J. Mol. Sci. 2023, 24, 7303. https://doi.org/10.3390/ijms24087303
Cieślik M, Zawadzka A, Czapski GA , Wilkaniec A, Adamczyk A. Developmental Stage-Dependent Changes in Mitochondrial Function in the Brain of Offspring Following Prenatal Maternal Immune Activation. Int. J. Mol. Sci. 2023, 24, 7243. https://doi.org/10.3390/ijms24087243
Palasz E, Wilkaniec A, Stanaszek L, Andrzejewska A, Adamczyk A. Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders. Int J Mol Sci. 2023, 24, 6321. https://doi.org/10.3390/ijms24076321
Gąssowska-Dobrowolska M, Kolasa A, Beversdorf DQ, Adamczyk A. Alterations in Cerebellar Microtubule Cytoskeletal Network in a ValproicAcid-Induced Rat Model of Autism Spectrum Disorders. Biomedicines. 2022 Nov 24;10(12):3031. doi: 10.3390/biomedicines10123031. PMID: 36551785; PMCID: PMC9776106.
Matuszewska M, Cieślik M, Wilkaniec A, Strawski M, Czapski GA. The Role of Bromodomain and Extraterminal (BET) Proteins in Controlling the Phagocytic Activity of Microglia In Vitro: Relevance to Alzheimer's Disease. Int J Mol Sci. 2022 Dec 20;24(1):13. doi: 10.3390/ijms24010013. PMID: 36613460; PMCID: PMC9820364.
Cieślik M, Gassowska-Dobrowolska M, Zawadzka A, Frontczak-Baniewicz M, Gewartowska M, Dominiak A, Czapski GA, Adamczyk A. The Synaptic Dysregulation in Adolescent Rats Exposed to Maternal Immune Activation. Front Mol Neurosci. 2021 Jan 14;13:555290. doi: 10.3389/fnmol.2020.555290. PMID: 33519375; PMCID: PMC7840660.
Gąssowska-Dobrowolska M, Kolasa-Wołosiuk A, Cieślik M, Dominiak A, Friedland K, Adamczyk A. Alterations in Tau Protein Level and Phosphorylation State in the Brain of the Autistic-Like Rats Induced by Prenatal Exposure to Valproic Acid. Int J Mol Sci. 2021 Mar 22;22(6):3209. doi: 10.3390/ijms22063209. PMID: 33809910; PMCID: PMC8004207.
Sun GY, Appenteng MK, Li R, Woo T, Yang B, Qin C, Pan M, Cieślik M, Cui J, Fritsche KL, Gu Z, Will M, Beversdorf D, Adamczyk A, Han X, Greenlief CM. Docosahexaenoic Acid (DHA) Supplementation Alters Phospholipid Species and Lipid Peroxidation Products in Adult Mouse Brain, Heart, and Plasma. Neuromolecular Med. 2021 Mar;23(1):118-129. doi: 10.1007/s12017-020-08616-0. Epub 2020 Sep 14. PMID: 32926329; PMCID: PMC9555299.
Strosznajder AK, Wójtowicz S, Jeżyna MJ, Sun GY, Strosznajder JB. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. Neuromolecular Med. 2021 Mar;23(1):86-98. doi: 10.1007/s12017-020-08629-9. Epub 2020 Nov 18. PMID: 33210212; PMCID: PMC7929960.
Motyl JA, Strosznajder JB, Wencel A, Strosznajder RP. Recent Insights into the Interplay of Alpha-Synuclein and Sphingolipid Signaling in Parkinson's Disease. Int J Mol Sci. 2021 Jun 11;22(12):6277. doi: 10.3390/ijms22126277. PMID: 34207975; PMCID: PMC8230587.
Jęśko H, Wieczorek I, Wencel PL, Gąssowska-Dobrowolska M, Lukiw WJ, Strosznajder RP. Age-Related Transcriptional Deregulation of Genes Coding Synaptic Proteins in Alzheimer's Disease Murine Model: Potential Neuroprotective Effect of Fingolimod. Front Mol Neurosci. 2021 Jul 9;14:660104. doi: 10.3389/fnmol.2021.660104. PMID: 34305524; PMCID: PMC8299068.
Wilkaniec A, Lenkiewicz AM, Babiec L, Murawska E, Jęśko HM, Cieślik M, Culmsee C, Adamczyk A. Exogenous Alpha-Synuclein Evoked Parkin Downregulation Promotes Mitochondrial Dysfunction in Neuronal Cells. Implications for Parkinson's Disease Pathology. Front Aging Neurosci. 2021 Feb 24;13:591475. doi: 10.3389/fnagi.2021.591475. PMID: 33716707; PMCID: PMC7943853.
Czapski GA, Babiec L, Jęśko H, Gąssowska-Dobrowolska M, Cieślik M, Matuszewska M, Frontczak-Baniewicz M, Zajdel K, Adamczyk A. Synaptic Alterations in a Transgenic Model of Tuberous Sclerosis Complex: Relevance to Autism Spectrum Disorders. Int J Mol Sci. 2021 Sep 17;22(18):10058. doi: 10.3390/ijms221810058. PMID: 34576223; PMCID: PMC8466868.
Czapski GA, Strosznajder JB. Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer's Disease. Int J Mol Sci. 2021 Oct 28;22(21):11677. doi: 10.3390/ijms222111677. PMID: 34769106; PMCID: PMC8584169.
Zawadzka A, Cieślik M, Adamczyk A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int J Mol Sci. 2021 Oct 26;22(21):11516. doi: 10.3390/ijms222111516. PMID: 34768946; PMCID: PMC8584025.
Czapski GA, Cieślik M, Białopiotrowicz E, Lukiw WJ, Strosznajder JB. Down-regulation of cyclin D2 in amyloid β toxicity, inflammation, and Alzheimer's disease. PLoS One. 2021 Nov 18;16(11):e0259740. doi: 10.1371/journal.pone.0259740. PMID: 34793515; PMCID: PMC8601534.
Gąssowska-Dobrowolska M, Cieślik M, Czapski GA, Jęśko H, Frontczak-Baniewicz M, Gewartowska M, Dominiak A, Polowy R, Filipkowski RK, Babiec L, Adamczyk A. Prenatal Exposure to Valproic Acid Affects Microglia and Synaptic Ultrastructure in a Brain-Region-Specific Manner in Young-Adult Male Rats: Relevance to Autism Spectrum Disorders. Int J Mol Sci. 2020 May 18;21(10):3576. doi: 10.3390/ijms21103576. PMID: 32443651; PMCID: PMC7279050.
Jęśko H, Cieślik M, Gromadzka G, Adamczyk A. Dysfunctional proteins in neuropsychiatric disorders: From neurodegeneration to autism spectrum disorders. Neurochem Int. 2020 Dec;141:104853. doi: 10.1016/j.neuint.2020.104853. Epub 2020 Sep 24. PMID: 32980494.
Wilkaniec A, Cieślik M, Murawska E, Babiec L, Gąssowska-Dobrowolska M, Pałasz E, Jęśko H, Adamczyk A. P2X7 Receptor is Involved in Mitochondrial Dysfunction Induced by Extracellular Alpha Synuclein in Neuroblastoma SH-SY5Y Cells. Int J Mol Sci. 2020 May 31;21(11):3959. doi: 10.3390/ijms21113959. PMID: 32486485; PMCID: PMC7312811.
Cieślik M, Czapski GA, Wójtowicz S, Wieczorek I, Wencel PL, Strosznajder RP, Jaber V, Lukiw WJ, Strosznajder JB. Alterations of Transcription of Genes Coding Anti-oxidative and Mitochondria-Related Proteins in Amyloid β Toxicity: Relevance to Alzheimer's Disease. Mol Neurobiol. 2020 Mar;57(3):1374-1388. doi: 10.1007/s12035-019-01819-y. Epub 2019 Nov 16. PMID: 31734880; PMCID: PMC7061023.
Cieślik M, Gąssowska-Dobrowolska M, Jęśko H, Czapski GA, Wilkaniec A, Zawadzka A, Dominiak A, Polowy R, Filipkowski RK, Boguszewski PM, Gewartowska M, Frontczak-Baniewicz M, Sun GY, Beversdorf DQ, Adamczyk A. Maternal Immune Activation Induces Neuroinflammation and Cortical Synaptic Deficits in the Adolescent Rat Offspring. Int J Mol Sci. 2020 Jun 8;21(11):4097. doi: 10.3390/ijms21114097. PMID: 32521803; PMCID: PMC7312084.
Jęśko H, Wencel PL, Wójtowicz S, Strosznajder J, Lukiw WJ, Strosznajder RP. Fingolimod Affects Transcription of Genes Encoding Enzymes of Ceramide Metabolism in Animal Model of Alzheimer's Disease. Mol Neurobiol. 2020 Jun;57(6):2799-2811. doi: 10.1007/s12035-020-01908-3. Epub 2020 Apr 30. PMID: 32356173; PMCID: PMC7253528.
Wilkaniec A, Lenkiewicz AM, Czapski GA, Jęśko HM, Hilgier W, Brodzik R, Gąssowska-Dobrowolska M, Culmsee C, Adamczyk A. Extracellular Alpha-Synuclein Oligomers Induce Parkin S-Nitrosylation: Relevance to Sporadic Parkinson's Disease Etiopathology. Mol Neurobiol. 2019 Jan;56(1):125-140. doi: 10.1007/s12035-018-1082-0. Epub 2018 Apr 21. PMID: 29681024; PMCID: PMC6334739.
Ganjam GK, Bolte K, Matschke LA, Neitemeier S, Dolga AM, Höllerhage M, Höglinger GU, Adamczyk A, Decher N, Oertel WH, Culmsee C. Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis. 2019 Nov 14;10(11):865. doi: 10.1038/s41419-019-2091-2. PMID: 31727879; PMCID: PMC6856124.
Jęśko H, Lukiw WJ, Wilkaniec A, Cieślik M, Gąssowska-Dobrowolska M, Murawska E, Hilgier W, Adamczyk A. Altered Expression of Urea Cycle Enzymes in Amyloid-β Protein Precursor Overexpressing PC12 Cells and in Sporadic Alzheimer's Disease Brain. J Alzheimers Dis. 2018;62(1):279-291. doi: 10.3233/JAD-170427. PMID: 29439324.
Wilkaniec A, Gąssowska-Dobrowolska M, Strawski M, Adamczyk A, Czapski GA. Inhibition of cyclin-dependent kinase 5 affects early neuroinflammatory signalling in murine model of amyloid beta toxicity. J Neuroinflammation. 2018 Jan 4;15(1):1. doi: 10.1186/s12974-017-1027-y. PMID: 29301548; PMCID: PMC5753486.
Wilkaniec A, Gąssowska M, Czapski GA, Cieślik M, Sulkowski G, Adamczyk A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells. Purinergic Signal. 2017 Sep;13(3):347-361. doi: 10.1007/s11302-017-9567-2. Epub 2017 May 17. PMID: 28516276; PMCID: PMC5563296.
Czapski GA, Cieślik M, Wencel PL, Wójtowicz S, Strosznajder RP, Strosznajder JB. Inhibition of poly(ADP-ribose) polymerase-1 alters expression of mitochondria-related genes in PC12 cells: relevance to mitochondrial homeostasis in neurodegenerative disorders. Biochim Biophys Acta Mol Cell Res. 2018 Feb;1865(2):281-288. doi: 10.1016/j.bbamcr.2017.11.003. Epub 2017 Nov 8. PMID: 29128369.
Jęśko H, Wencel P, Strosznajder RP, Strosznajder JB. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem Res. 2017 Mar;42(3):876-890. doi: 10.1007/s11064-016-2110-y. Epub 2016 Nov 24. PMID: 27882448; PMCID: PMC5357501.
Jęśko H, Lenkiewicz AM, Adamczyk A. Treatments and compositions targeting α-synuclein: a patent review (2010-2016). Expert Opin Ther Pat. 2017 Apr;27(4):427-438. doi: 10.1080/13543776.2017.1261112. Epub 2016 Nov 23. PMID: 27841042.
Czapski GA, Gąssowska M, Wilkaniec A, Chalimoniuk M, Strosznajder JB, Adamczyk A. The mechanisms regulating cyclin-dependent kinase 5 in hippocampus during systemic inflammatory response: The effect on inflammatory gene expression. Neurochem Int. 2016 Feb;93:103-12. doi: 10.1016/j.neuint.2016.01.005. Epub 2016 Jan 21. PMID: 26806339.
Czapski GA, Czubowicz K, Strosznajder JB, Strosznajder RP. The Lipoxygenases: Their Regulation and Implication in Alzheimer's Disease. Neurochem Res. 2016 Feb;41(1-2):243-57. doi: 10.1007/s11064-015-1776-x. Epub 2015 Dec 16. PMID: 26677076; PMCID: PMC4773476.
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Tarnowski M, Pilutin A, Gutowska I, Strużyńska L, Chlubek D, Adamczyk A. Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3β and CDK5 dependent manner: Relevance to neurological disorders. Toxicology. 2016 Mar 10;347-349:17-28. doi: 10.1016/j.tox.2016.03.002. Epub 2016 Mar 21. PMID: 27012722.
Jęśko H, Wilkaniec A, Cieślik M, Hilgier W, Gąssowska M, Lukiw WJ, Adamczyk A. Altered Arginine Metabolism in Cells Transfected with Human Wild-Type Beta Amyloid Precursor Protein (βAPP). Curr Alzheimer Res. 2016;13(9):1030-9. doi: 10.2174/1567205013666160314150348. PMID: 26971935.
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Frontczak-Baniewicz M, Gewartowska M, Strużyńska L, Gutowska I, Chlubek D, Adamczyk A. Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring. Toxicology. 2016 Dec 12;373:13-29. doi: 10.1016/j.tox.2016.10.014. Epub 2016 Oct 29. PMID: 27974193.
Wilkaniec A, Czapski GA, Adamczyk A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J Neurochem. 2016 Jan;136(2):222-33. doi: 10.1111/jnc.13365. Epub 2015 Oct 14. PMID: 26376455.